New DNA editing technique for mitochondrial disease may render three parent embryos redundant
A new technique for removing harmful genes from strands of DNA could potentially obviate the need for three parent embryos for preventing mitochondrial disease.
Researchers from the Salk Institute for Biological Studies in La Jolla, California, have reported success for the first time in using gene-editing technology to prevent mitochondrial diseases being passed from female mice to their offspring.
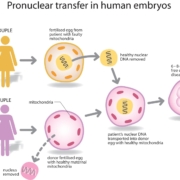
Mitochondrial diseases are inherited maternally and cause a variety of severe conditions that currently have no cure. The UK government has recently legalised the controversial use of embryos carrying DNA from three genetic parents to prevent their transmission, but the proposed techniques have been criticised on both ethical and safety grounds (see my previous review).
This new research, published in the 23 April edition of Cell magazine, involves injecting affected embryos with RNA which leads to the production of enzymes which specifically target and remove faulty genes.
It is reported on this week in Nature, Popular Science, Medical News Today, Tech Times, and (amazingly) has even come to the attention of the BBC. Ted Morrow’s blog gives a useful overview.
Authors Alejandro Ocampo and Juan Carlos Izpisua Belmonte realised that reducing the amount of mutant mitochondrial DNA in an egg or fertilised embryo could reduce the chance of mitochondrial disease developing.
They achieved this by injecting mouse embryos with a segment of RNA designed to produce a DNA-cutting enzymes called restriction endonucleases and transcription activator-like effector nucleases (TALENs).
These enzymes then sought out mitochondria with mutated DNA and removed it while leaving the normal mitochondrial DNA intact.
The treated embryos were then transferred to female mice where they developed normally and resulted in healthy pups with low levels of the targeted mitochondrial DNA.
These pups later went on to give birth to healthy offspring themselves, demonstrating that this is a viable approach for preventing transgenerational transmission of mitochondrial diseases.
The researchers then trialled the TALENs using mouse eggs that contained genetic material from human patients mitochondrial DNA mutations known to cause two disorders – Leber’s hereditary optic neuropathy and dystonia (LHOND) and neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP).
Again, the technique resulted in a significant reduction of the mutated DNA.
It is early days with this new technique which will need thorough testing in mice and non-human primates before being ready for testing in humans.
However it has several obvious advantages over controversial three parent embryo techniques.
First, it does not require DNA donation and so avoids the health risks to donors (such as OHSS) associated with egg harvesting.
Second, it does not involve cell nuclear replacement (cloning) technology with all its safety concerns.
Third, it can be tested on eggs as well as embryos and does not involve the destruction of existing embryos.
Fourth, it is technically much easier to perform than the mitochondrial (or more accurately cytoplasmic) replacement used in three parent techniques. RNA injection is a technique that can be carried out relatively easily in IVF clinics.
Fifth, it appears to be a far more finely targeted technique, eliminating faulty genes rather than replacing the whole cytoplasm with all of it mitochondrial and other organelles. More like a sniper than a blunderbuss or carpet bomber.
Finally it does not produce offspring with three genetic parents.
However, we are still left with three concerns.
First, this new technique does still involve genetic medication of the germline, and the possibility that any ‘mistakes’ in editing would be passed on down the generations. This has already led David King of Human Genetics Alert to reject it outright.
Second, we cannot be sure, without a lot of further research, just how finely tuned it is as an editing tool. How much damage might be done to other genes in the vicinity and what effect might this have?
Third, once this tool is more widely available, might it then be used by unscrupulous researchers or scientists to edit DNA in more dangerous contexts?
However, were it to work as well as some are hoping, it could potentially reduce the three parent technique, over which so much time, money and sweat has been expended, to a tiny historical footnote.
It will be intriguing to see how this research progresses and how long it takes for those British science journalists and parliamentarians who have been pushing three parent embryos so aggressively to take notice.






Leave a Reply
Want to join the discussion?Feel free to contribute!